Last Friday my wife and I were driving through the wilds of Utah (aside: wow, is the West different from The Hub of the Universe!) when we chanced upon SciFri, which was doing a great segment about cool government-funded energy research (no, not the Solindra picking winners-type stuff, but real basic research that can lead to quantum leaps in performance).
One of the speakers was Prof. Jennifer Lewis, who has the all-time greatest academic title: Hansjörg Wyss Professor of Biologically Inspired Engineering at the Harvard School of Engineering and Applied Sciences! Go biomimicry (just a little reminder, BTW, that nature has already solved every problem that we, as an advanced, information-based economy, face. Think not? The answer to your problem lies just outside your window: we’re just too divorced from nature to be able to see it!)!
OK, got that out of my system…..
Now for the big news: Prof. Lewis’ team and their associates at the University of Illinois have invented the Holy Grail for Internet of Things sensors: lithium-ion batteries the size of a grain of sand, created through 3-D printing (as you may remember, I blogged recently about the role 3-D printing could play in fully-realizing the IoT’s potential. Little did I think it would be this soon, and this direct a role)!
This is a game-changer when it comes to sensors: their size has been getting smaller and smaller, but the big obstacle to realizing Kristofer Pister’s vision of “smart dust” sensors so tiny and self-powered that they could be strewn about was that the batteries were still relatively big and clunky. Lewis’ breakthrough changes all of that.
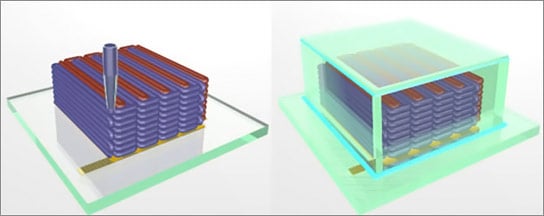
lithium-ion batteries produced by 3-D printing
The batteries are built by printing precisely interlaced stacks of tiny battery electrodes, each less than the diameter of a human hair.
Here’s the process:
“In this case, the inks also had to function as electrochemically active materials to create working anodes and cathodes, and they had to harden into layers that are as narrow as those produced by thin-film manufacturing methods. To accomplish these goals, the researchers created an ink for the anode with nanoparticles of one lithium metal oxide compound, and an ink for the cathode from nanoparticles of another. The printer deposited the inks onto the teeth of two gold combs, creating a tightly interlaced stack of anodes and cathodes. Then the researchers packaged the electrodes into a tiny container and filled it with an electrolyte solution to complete the battery.”
The research was funded by the National Science Foundation and the DOE Energy Frontier Research Center on Light-Material Interactions in Energy Conversion.
This is so exciting. Now to commercialize the technology and to turn our attention to the real obstacles to the Internet of Things: privacy and security problems!
